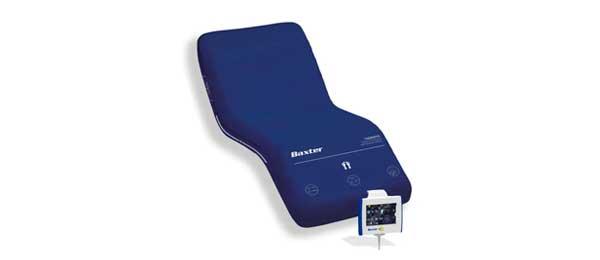
Overview
The P290 mattress overlay, or mattress replacement system was designed to help prevent and treat pressure ulcers for moderate-risk patients.¹
The P290 surface provides immersion and envelopment through a choice of Alternating and Continuous Low Pressure therapy modes.
The ALP And CLP options are aligned with the NPIAP/EPUAP/PPPIA International Guidelines which advise clinicians to change from a standard support surface to a specialty support surface (i.e. alternating pressure air mattress, low-air-loss mattress, or air fluidized bed) when the individual:²
- Cannot be positioned off the existing pressure ulcer
- Has a pressure ulcer on two or more turning surfaces, which limits repositioning positions
- Has a pressure ulcer that fails to heal or deteriorates despite appropriate comprehensive care
- Is at risk for additional pressure ulcers
- Is uncomfortable
- “Bottoms out” (i.e. touches the bed frame) on the current support surface
Features
- Specifically designed for enhanced prevention and treatment of patients at risk or suffering from pressure ulcers
- A choice of alternating or continuous low pressure therapiesto support differing patient needs and clinical preferences
- Adjustable cycle times and firmness setting for control over patient therapy and comfort
- Patient and caregiver safety features including a automatic control panel lock-out, fault indicators and 2-hour transport mode
- Two pressure-redistributing zones provide additional protection for vulnerable body areas, including 5° sloped alternating heel zone
- P-MAX and PLUS inflation designed to offer additional support during patient positioning and transfer activities
- Mattress replacement is available with either an air or foam patient support layer
- X-ray sleeve to aid in bed x-rays.*
- Evacuation handles to assist with moving patients during an emergency.*
*Optional
Education & Documents
Please visit our manuals & technical documents center.
Related Products
For safe and proper use of the product mentioned herein, please refer to the appropriate Operator’s Manual or Instructions for Use.
This medical device is a regulated health product which, pursuant to such regulation, bears a CE mark. Baxter recommends that you carefully read the detailed instructions for safe and proper use included in the documents accompanying the medical devices.
The personnel of healthcare establishments are responsible for the proper use and maintenance of this medical device.