
Overview
Meet a Smart, Dynamic Mattress Designed for Today’s Healthcare Environments
The Therapy2 dynamic surface is a standalone full-air surface with smart features such as connectivity, patient exit alerts and continous monitoring* among others. This therapeutic surface has integrated pulmonary, pressure ulcer prevention1-4 and prone features* designed with the most vulnerable patients in mind. It can be part of the hospital protocols to assist in the prevention of pressure ulcers (PU)3,4 and pulmonary complications associated with immobility.1-2 It is ideally suited for Intensive Care (ICU) and Step-down (SDU) units .
Put Safety & Comfort First
This dynamic air pressure surface is designed for comfort and ready to support skin protection and pulmonary specialized therapies** if complications arise.
Work Efficiently
The therapeutic** and smart*** features help to release time for your busy staff, so they can streamline efficiencies and react to patient needs more quickly.****
Keep Connected
Stay informed on patient and/or surface status5, and react to patient needs more quickly.
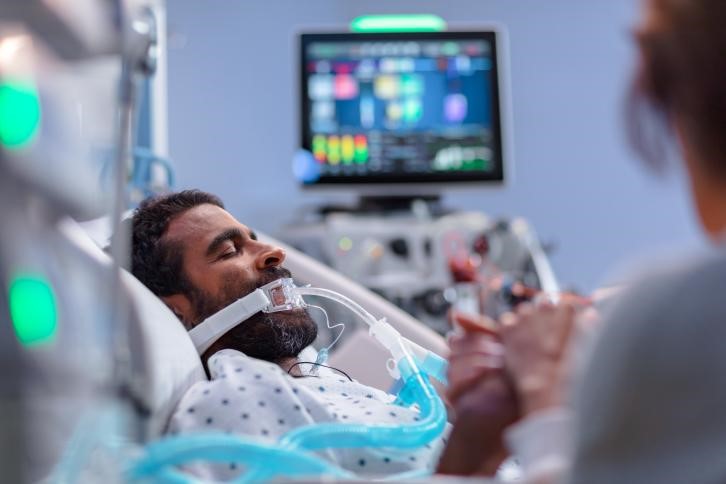
Support Pulmonary Therapies
Implement pulmonary therapies for patients without adding strain to your caregivers.
- Provide continuous lateral rotation therapy (CLRT) to help prevent pulmonary complications1-2
- Prone position management* helps caregivers to effectively manage patients in the prone position even during intubation6
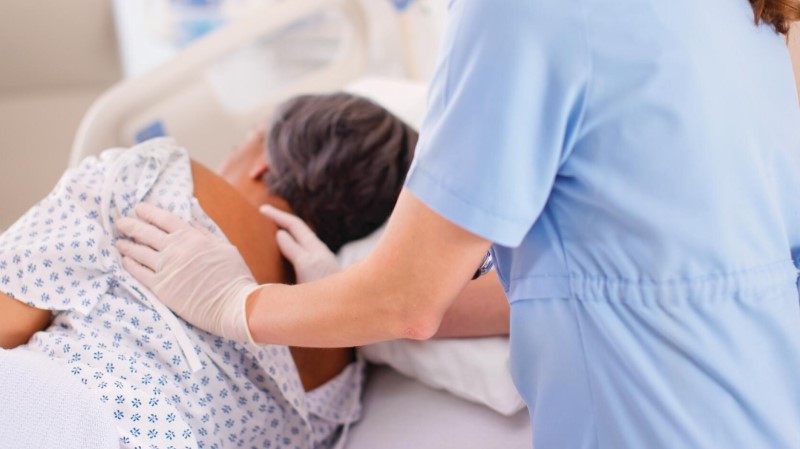
Assist in the Prevention and Treatment of Pressure Ulcers3-4
Immobile patients are at higher risk for skin breakdown.7 The Therapy2 dynamic surface is designed for advanced skin protection, so you can:
- Assist in the prevention and treatment of pressure ulcers with Continuous Low Pressure (CLP) or Alternating Low Pressure (ALP) therapy modes
- Adjust air pressure with Comfort Adjust technology
- Help turn patients easily with patient mobilization mode and reminders achieving up to 36% reduction in effort required to turn patient8
- Manage heat and moisture at the interface between the patient’s body and the surface with MicroClimate Management*9-12

Be Alerted to Physiological Indicators of a Patient's Condition13
As a second set of eyes, the contact-free, continuous monitoring system* acts as a complimentary to episodic and continuous vital signs monitors and alerts the caregiver of heart or respiratory rates that exceed the pre-determined thresholds.
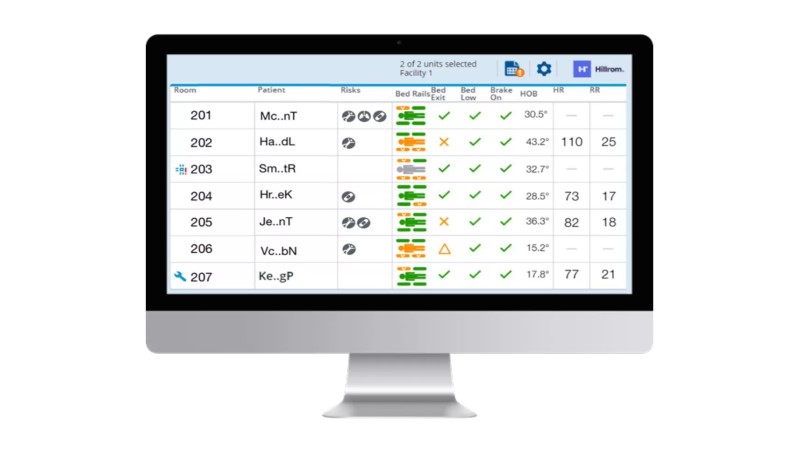
Stay Connected to Patient and Surface Status
Allow the caregiver to stay connected to be informed on patient and surface status5 — so that they can react to patient needs faster.
- Timely alerts — patient exit, HR and/or RR thresholds*, and turn reminders — to bring clinicians to the bedside for faster interventions
- Connectivity* to send clinical data5 from the surface to the nurse’s station or EMR
Education & Documentation
Please visit our manuals & technical documents center
Related Products
* Optional feature. Please check availability with your local Baxter representative.
** Therapy2 is a therapeutic mattress with continuous low pressure (CLP), alternating low pressure (ALP) and MCM modes. There is also an aid to prevent pulmonary complications by continuous lateral rotation.
***Some of the smart features are: I-mmersion sensor, patient exit alerts, patient and surface notifications, continuous monitoring* and connectivity capabilities.
****Therapy2 helps release time and streamline efficiencies through assisting in the prevention of PU3,4 and pulmonary complications associated with immobility1-2, helping to move the patient by lateral rotation, eliminating the need for staff to find, install and clean the air pump, allowing to perform in-room portable X-ray while the patient is lying on the surface with the X-ray cassette sleeve, among others.
Manufacturer: Hill-Rom S.A.S. - Z.I. du Talhouët 56330 Pluvigner, France
Class IIa - Notified Body GMED 0459
This medical device is a regulated health product which, pursuant to such regulation bears a CE mark. Baxter recommends that you carefully read the detailled instructions for safe and proper use included in the documents accompanying the medical device. The personnel of healthcare establishments are responsible for the proper use and maintenance of these medical devices.
PMM No.: EMA-CS394-240010 (v 1) 09/2024
